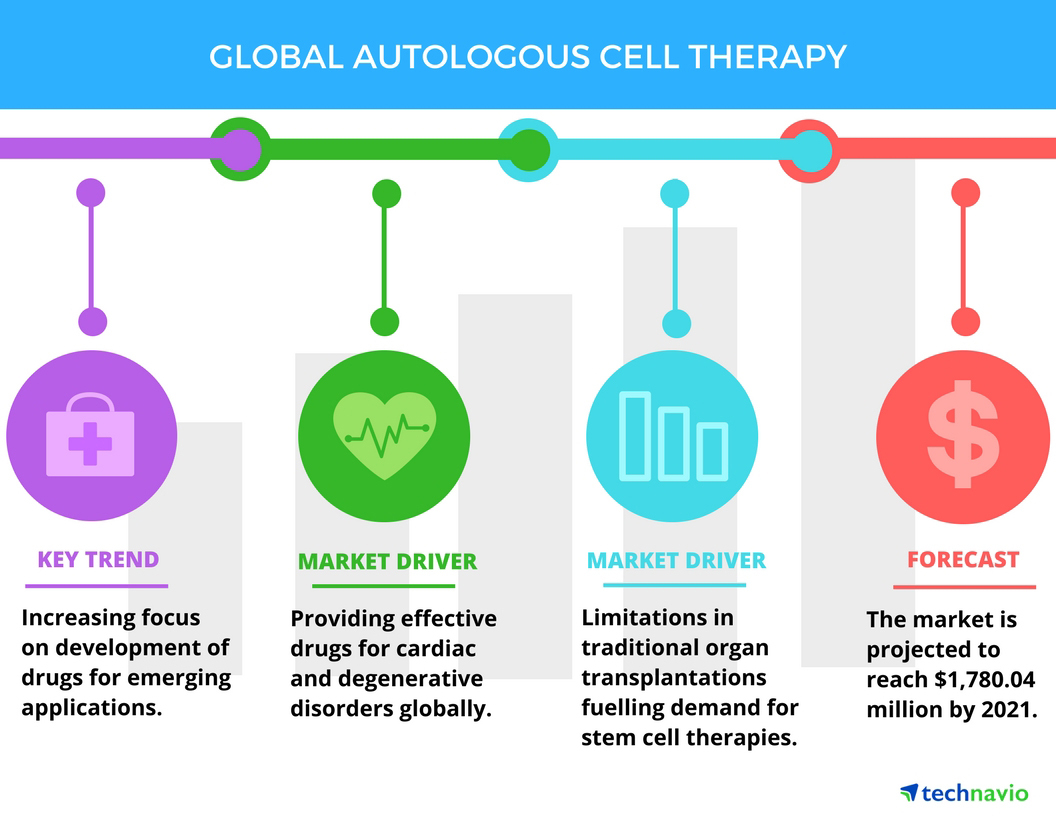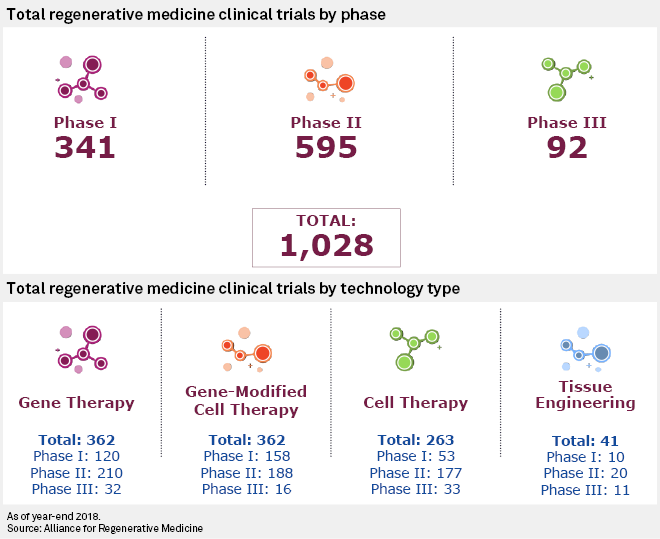
Drug companies chase deals for cell and gene therapies, biotech's 'next wave' | S&P Global Market Intelligence

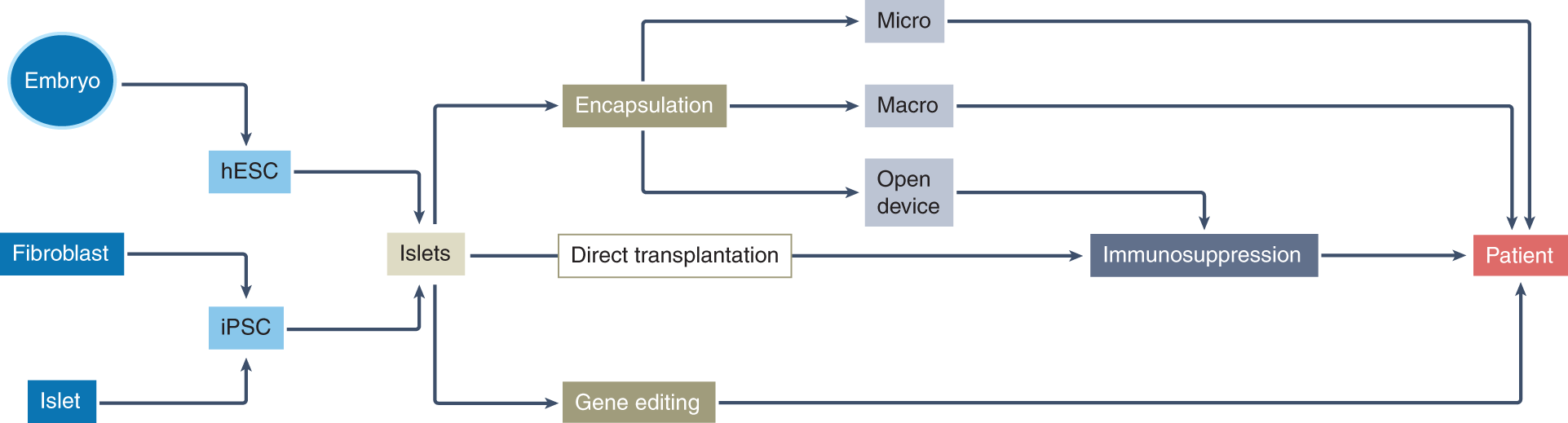
Gene-and cell-based therapy landscape. CTMP, cell therapy medicinal... | Download Scientific Diagram
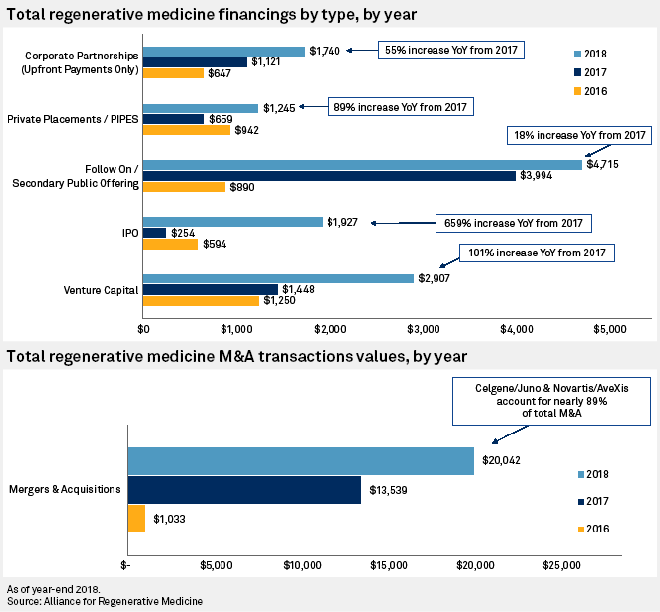
Drug companies chase deals for cell and gene therapies, biotech's 'next wave' | S&P Global Market Intelligence
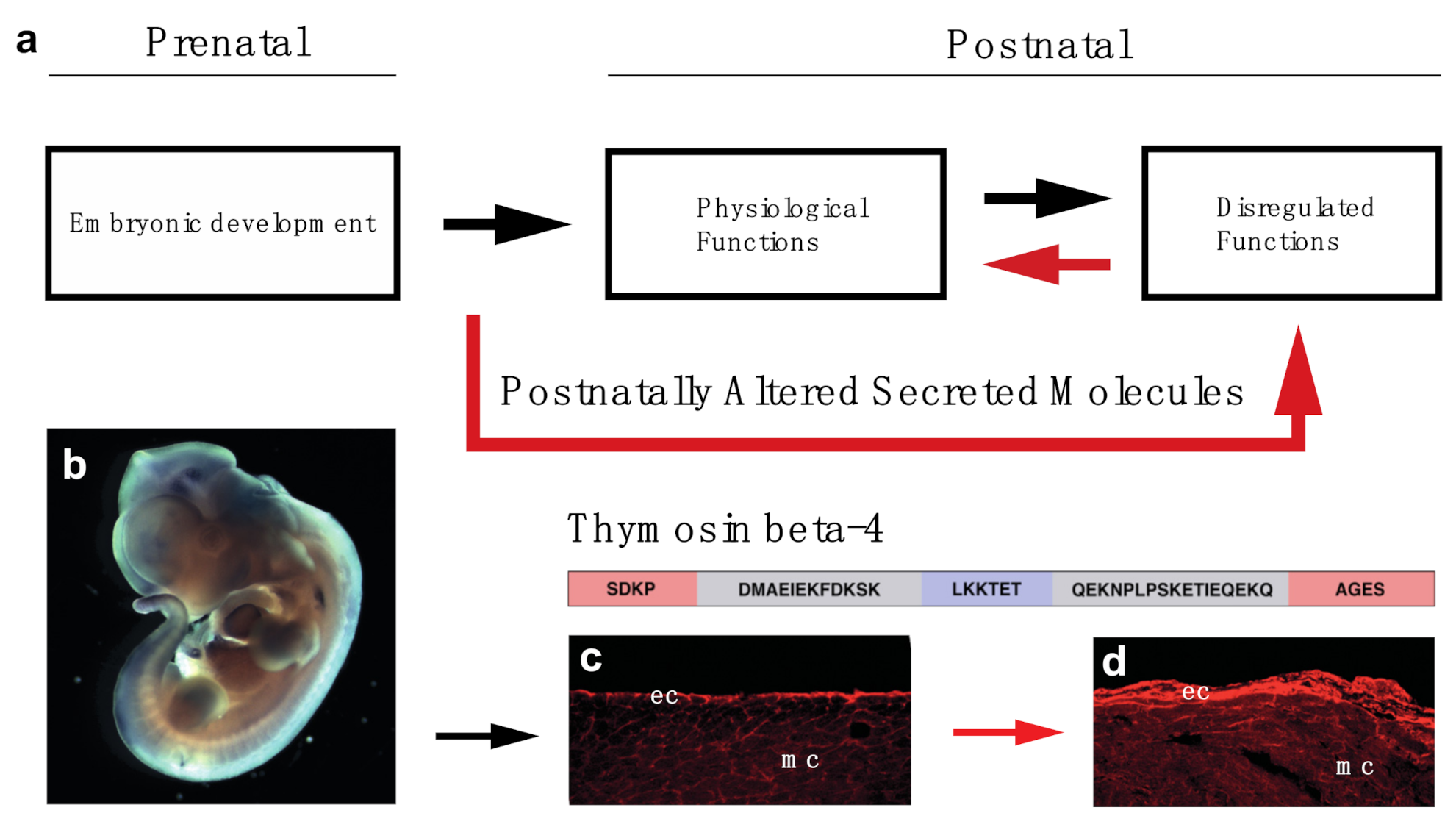
Cells | Free Full-Text | Utilizing Developmentally Essential Secreted Peptides Such as Thymosin Beta-4 to Remind the Adult Organs of Their Embryonic State—New Directions in Anti-Aging Regenerative Therapies | HTML

Cell Therapy Market Key Competitors are | Fibrocell Science Inc., Cellectis, BioNTech IMFS, pluristem, Grupo Praxis, Genzyme Corporation, Advanced Tissue, Cells for Cells and others | Medgadget
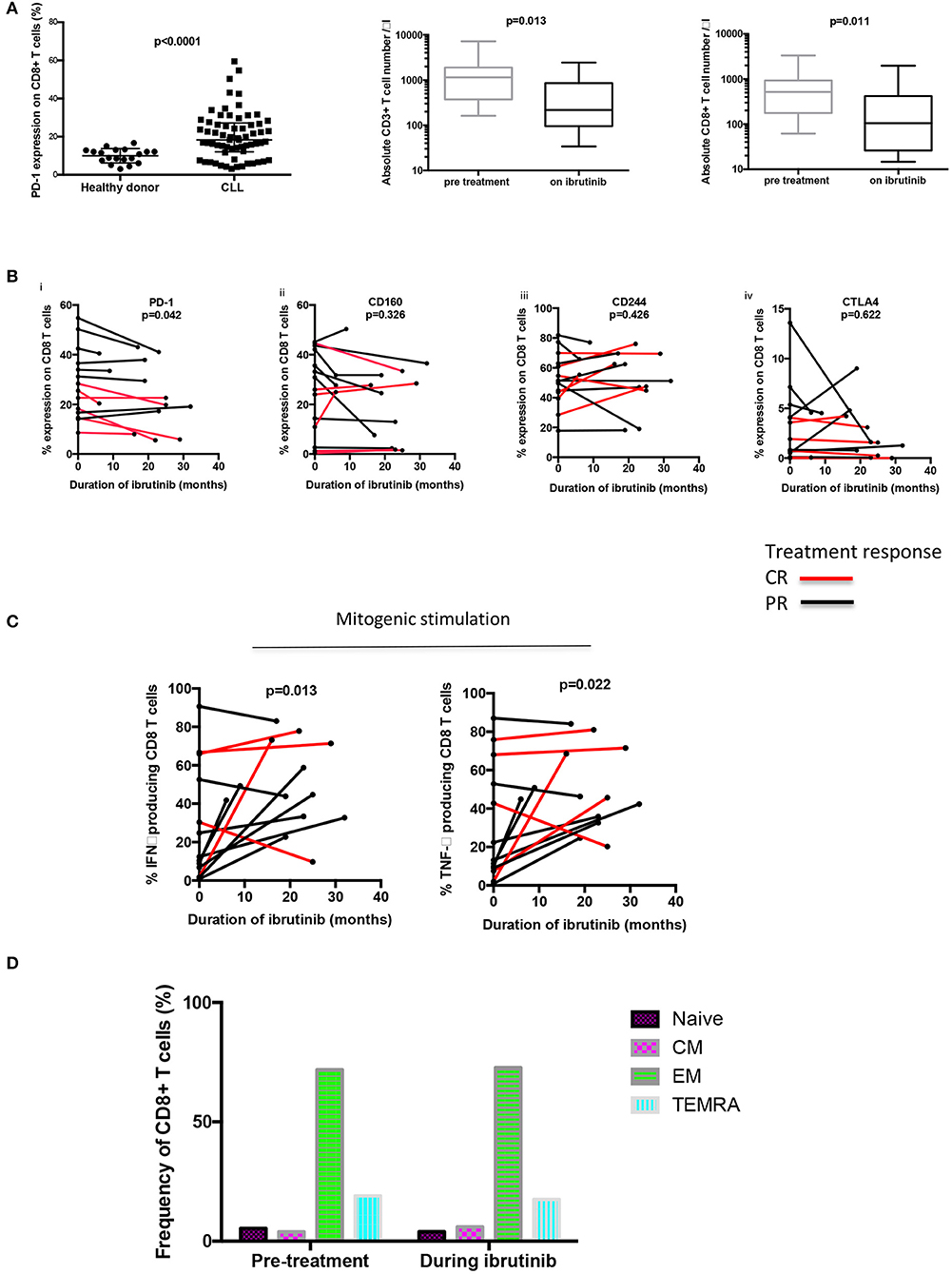
Frontiers | Long-Term Ibrutinib Therapy Reverses CD8+ T Cell Exhaustion in B Cell Chronic Lymphocytic Leukaemia

The Potential Application of Real‑Time Release Testing for the Biomanufacture of Autologous Cell‑Based ImmunotherapiesBioProcess International

Unbiased in vivo preclinical evaluation of anticancer drugs identifies effective therapy for the treatment of pancreatic adenocarcinoma | PNAS
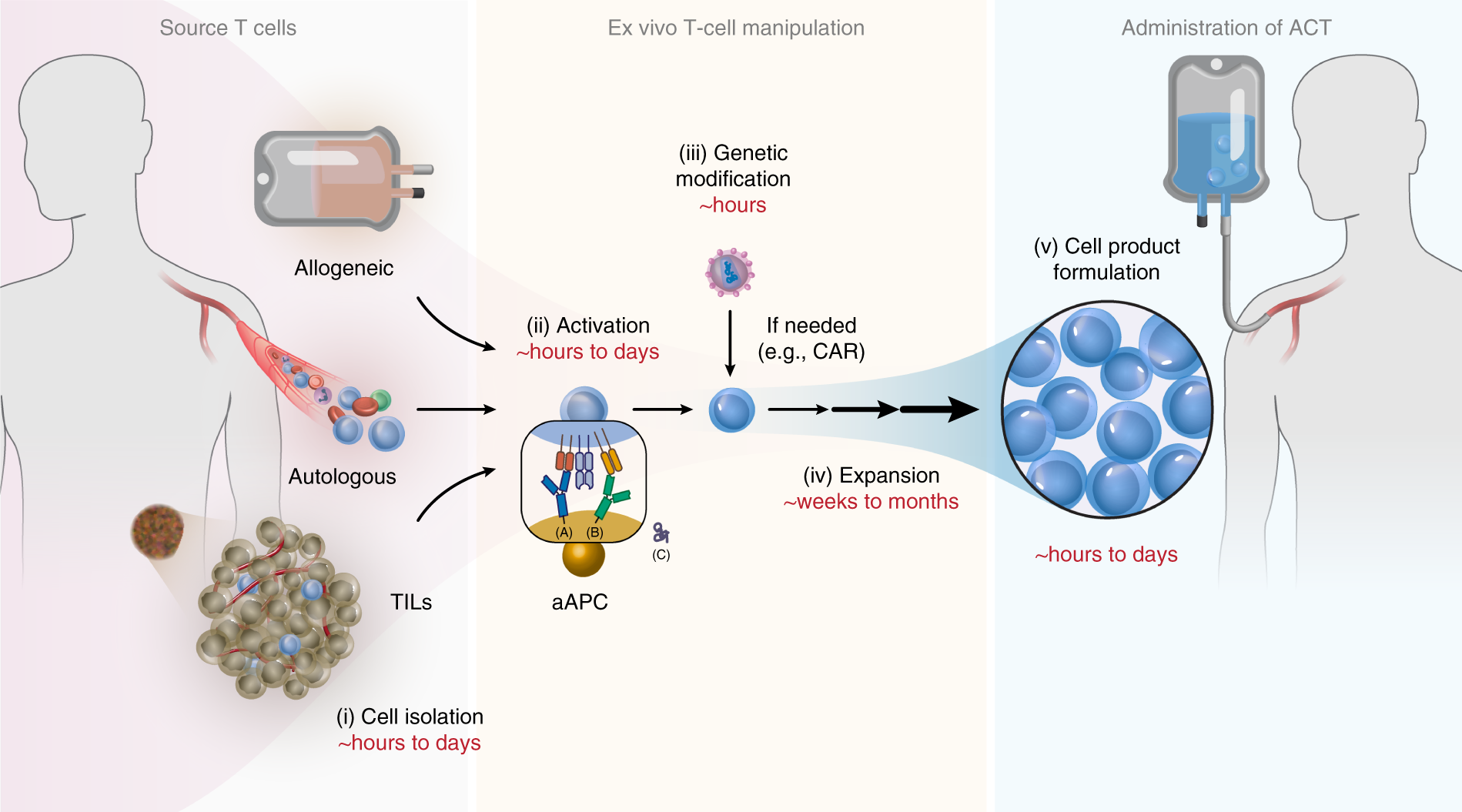
Activation and expansion of human T cells using artificial antigen-presenting cell scaffolds | Nature Protocols
Cell Therapeutics annuncia la chiusura dell'offerta pubblica sottoscritta di USD 60 milioni di Azioni Privilegiate convertibili