Subjective endpoints in clinical trials: the case for blinded independent central review - Document - Gale Academic OneFile

Challenging Issues in Clinical Trial Design: Part 4 of a 4-Part Series on Statistics for Clinical Trials - ScienceDirect
Inadequate planning and reporting of adjudication committees in clinical trials: recommendation proposal

Rationale and design of a prospective substudy of clinical endpoint adjudication processes within an investigator-reported randomised controlled trial in patients with coronary artery disease: the GLOBAL LEADERS Adjudication Sub-StudY (GLASSY) | BMJ
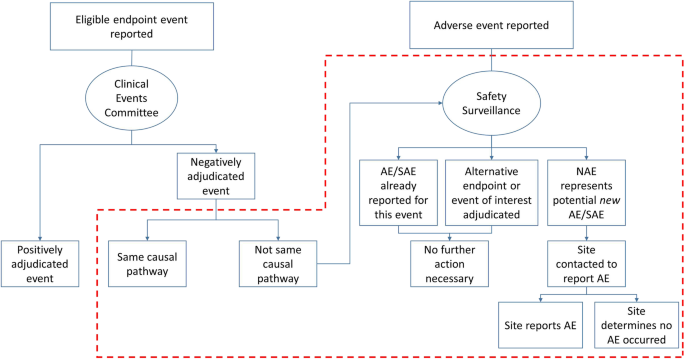
Methods for safety and endpoint ascertainment: identification of adverse events through scrutiny of negatively adjudicated events | Trials | Full Text

Clinical endpoint adjudication in a contemporary all-comers coronary stent investigation: Methodology and external validation - ScienceDirect