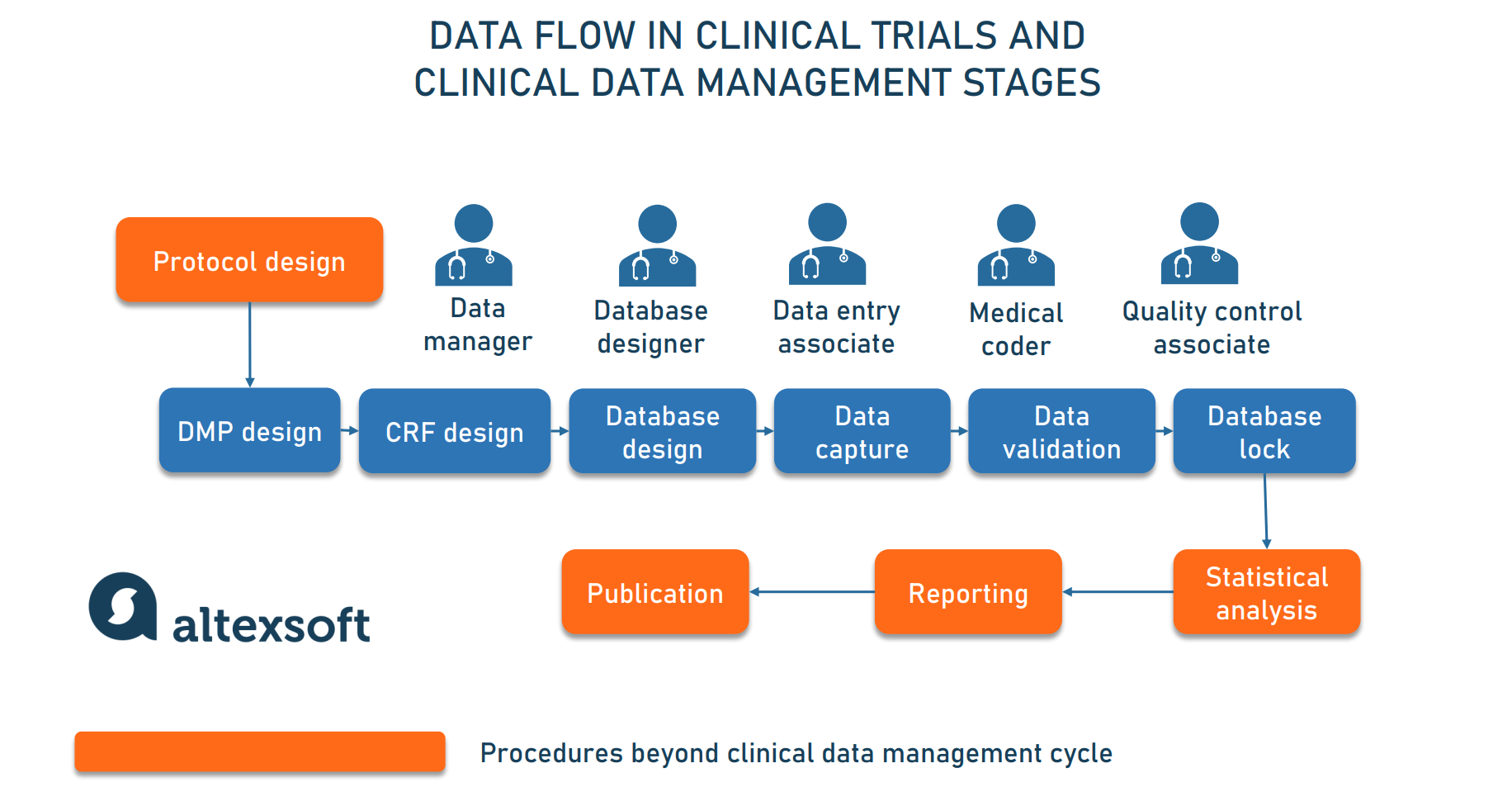
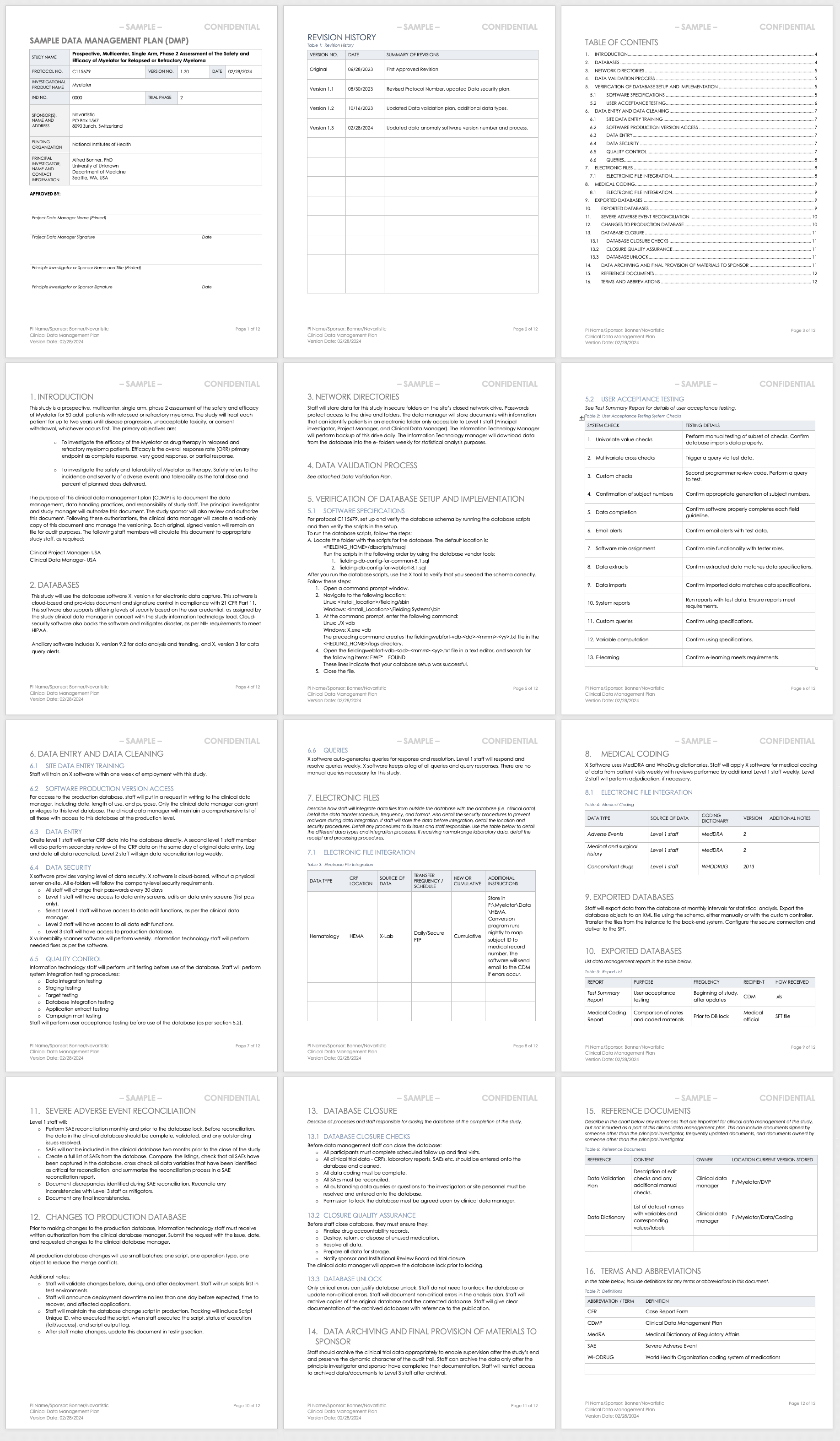
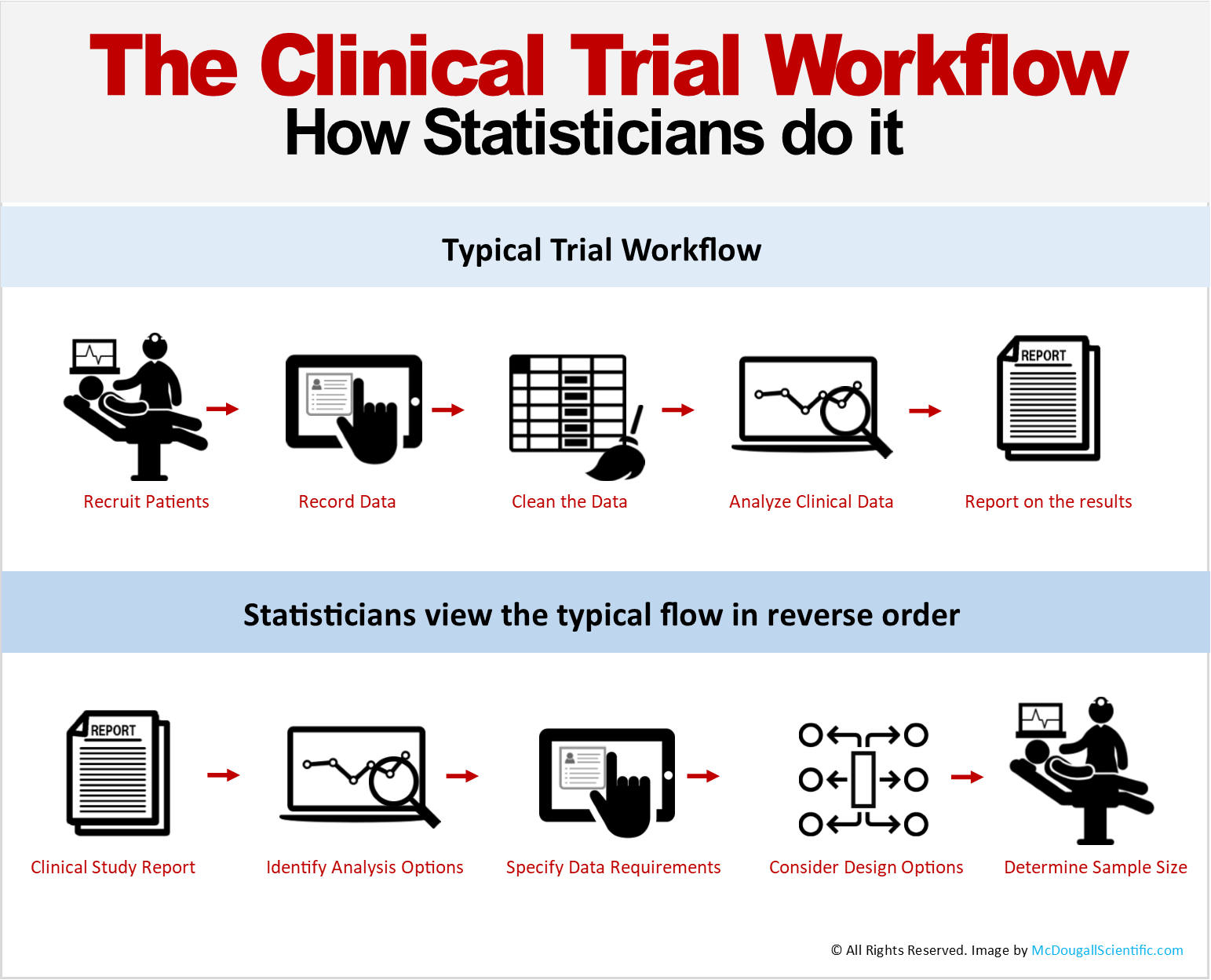
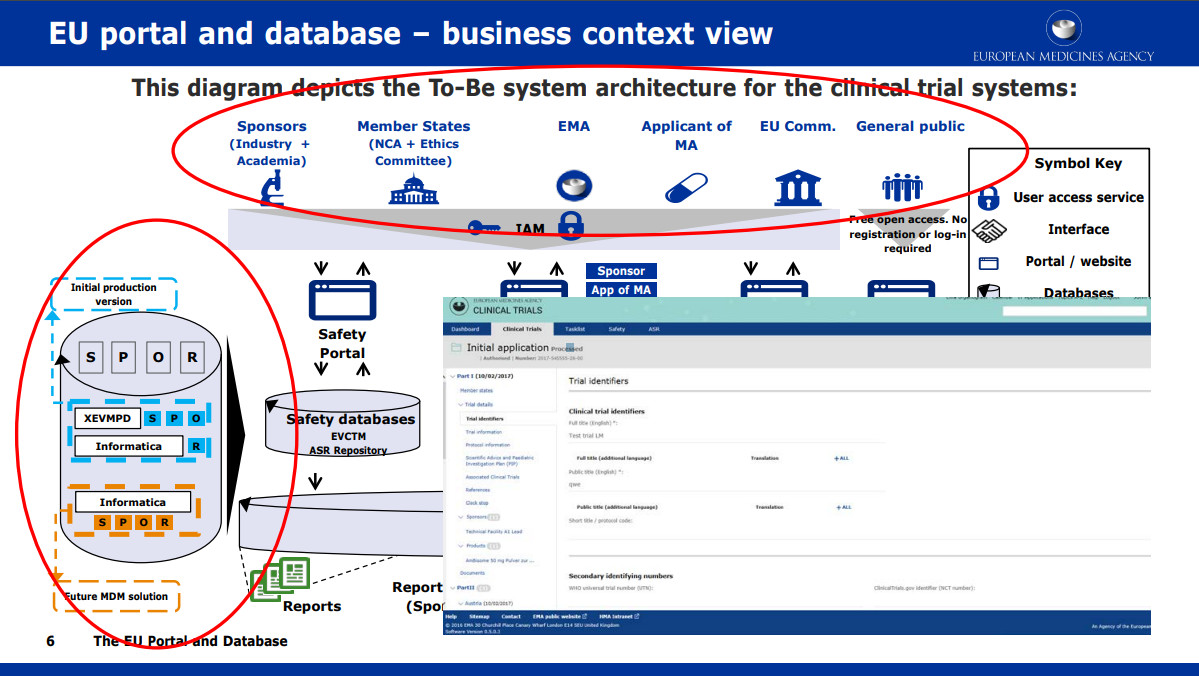
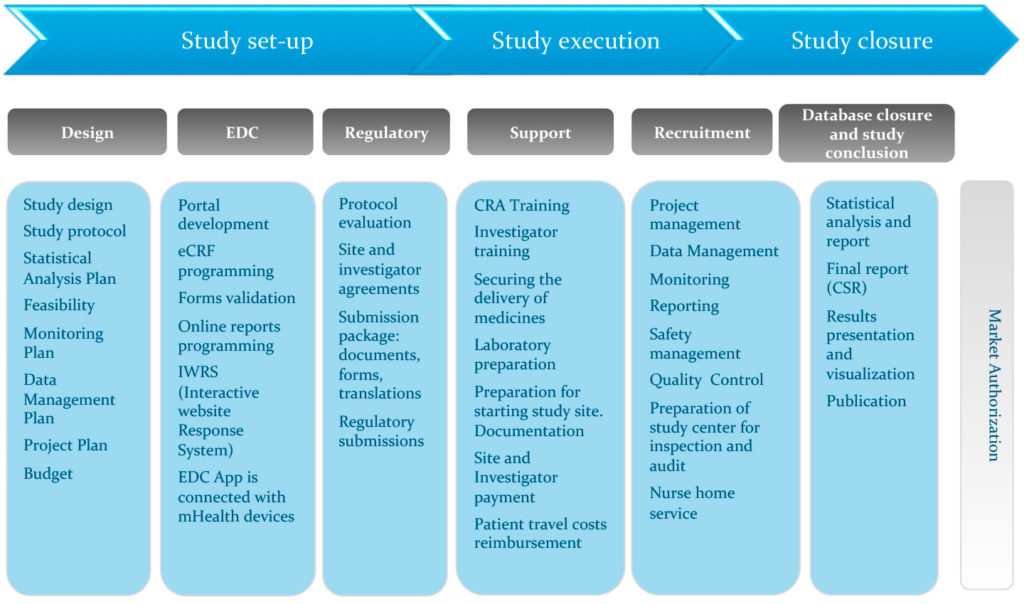
A schematic representation of the database for Aggregate Analysis of... | Download Scientific Diagram
![PDF] A database system for integrated clinical trial management, control, statistical analysis and ICH-compliant reporting | Semantic Scholar PDF] A database system for integrated clinical trial management, control, statistical analysis and ICH-compliant reporting | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/b7222080945658c3980e16ccdbbceead72486f32/2-Figure1-1.png)
PDF] A database system for integrated clinical trial management, control, statistical analysis and ICH-compliant reporting | Semantic Scholar
![PDF] An OMOP CDM-Based Relational Database of Clinical Research Eligibility Criteria | Semantic Scholar PDF] An OMOP CDM-Based Relational Database of Clinical Research Eligibility Criteria | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/78cbb6c6ecddffa5d21a86c3eaf862849b636105/10-Figure1-1.png)
PDF] An OMOP CDM-Based Relational Database of Clinical Research Eligibility Criteria | Semantic Scholar