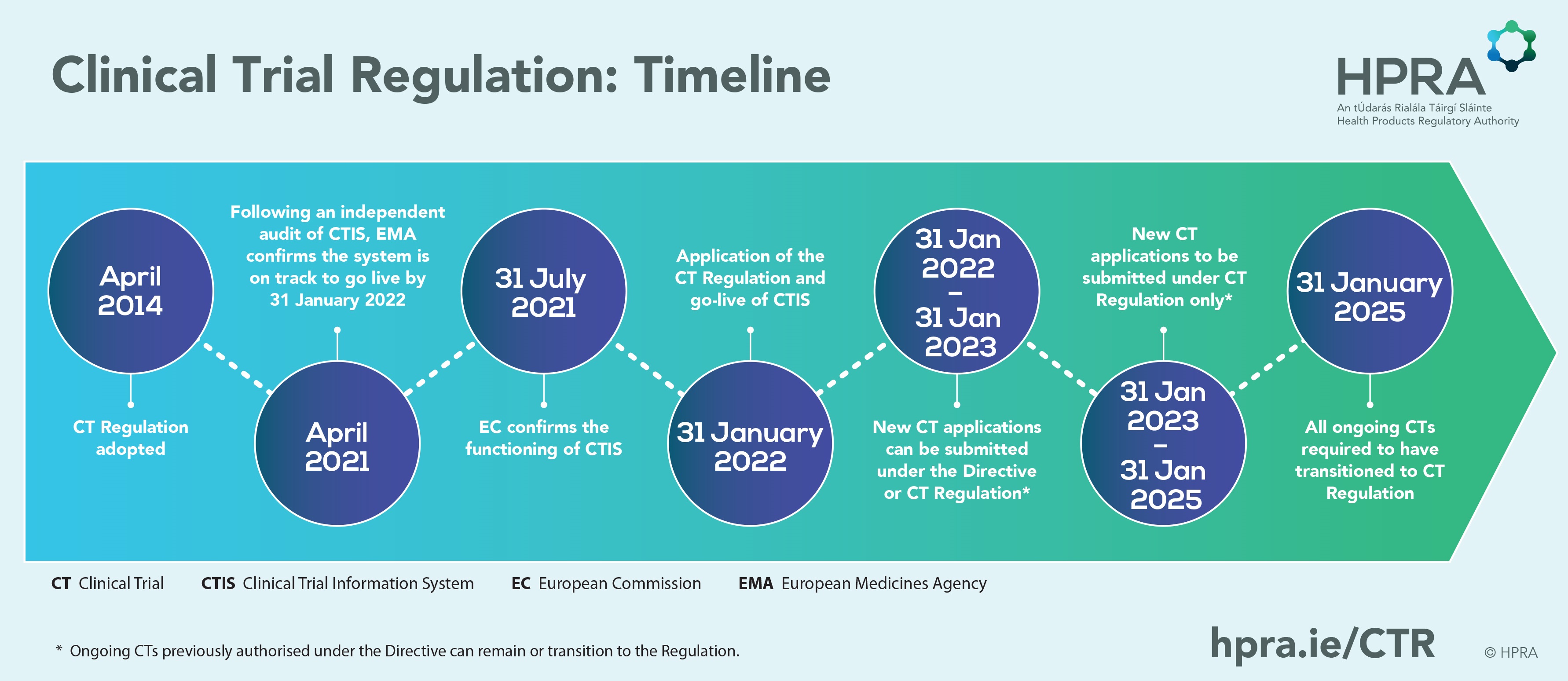
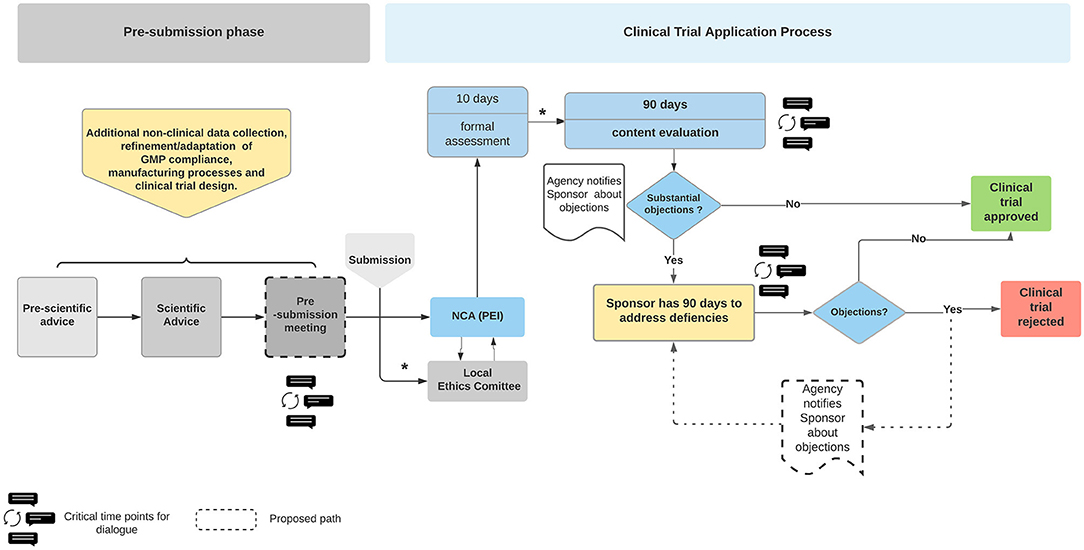
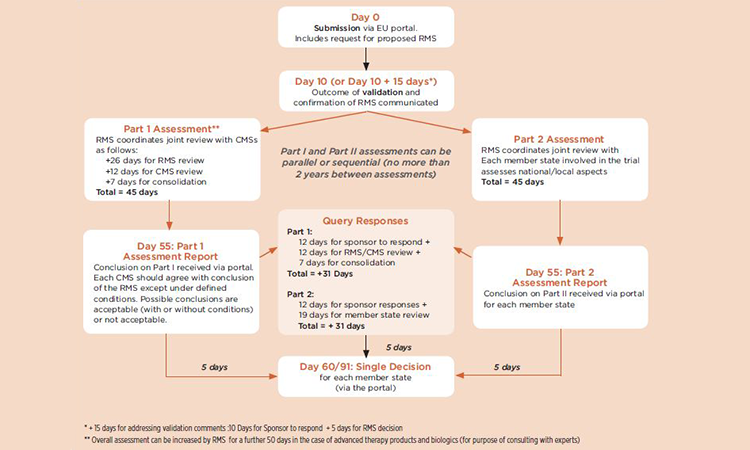
Commentary on the EMA Guideline on strategies to identify and mitigate risks for first‐in‐human and early clinical trials with investigational medicinal products - Gerven - 2018 - British Journal of Clinical Pharmacology -

When innovation outpaces regulations: The legal challenges for direct‐to‐patient supply of investigational medicinal products - Malone - 2022 - British Journal of Clinical Pharmacology - Wiley Online Library
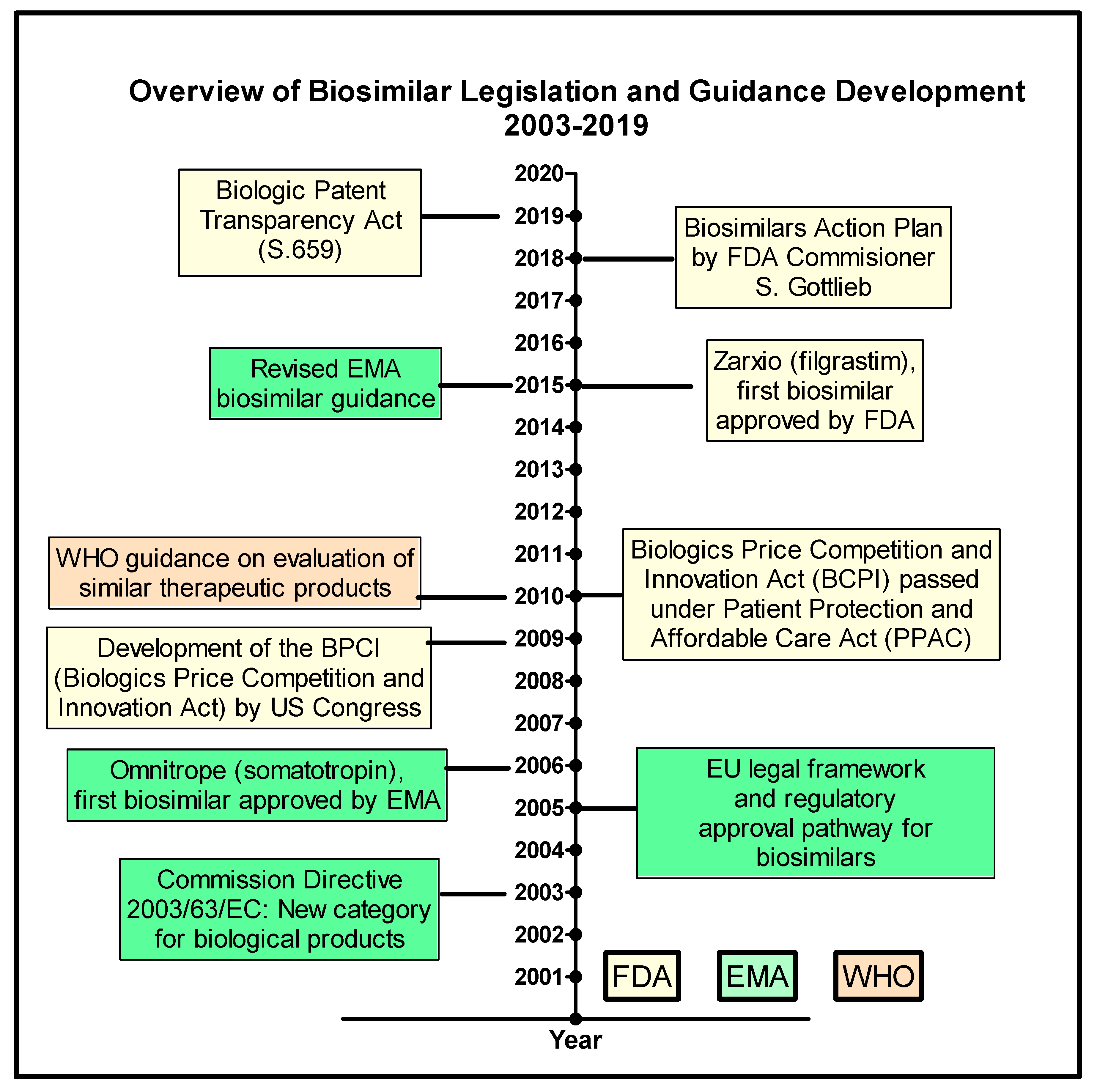
Pharmaceutics | Free Full-Text | The Biosimilar Landscape: An Overview of Regulatory Approvals by the EMA and FDA | HTML
Frontiers | Transitioning From Preclinical Evidence to Advanced Therapy Medicinal Product: A Spanish Experience

EMA & FDA Approvals and Recommendations in 2020 for Oncology Drugs and Diagnostics/Devices | CATO SMS
Overview of comments - Requirements to the chemical and pharmaceutical quality documentation concerning investigational medicina
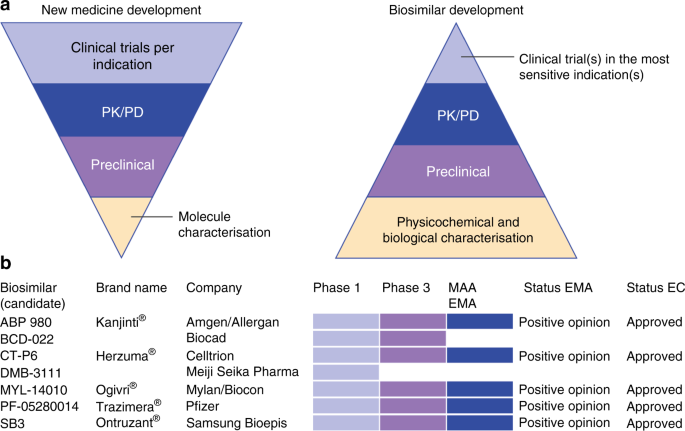
The arrival of biosimilar monoclonal antibodies in oncology: clinical studies for trastuzumab biosimilars | British Journal of Cancer

Tag | CRO, Clinical, TMF, eTMF, trial master file, inspection, audit, audit ready, inspection ready, Clinical Research Organisation, regulation, EU, EMA, MHRA, Andy FIsher | Pharma IQ