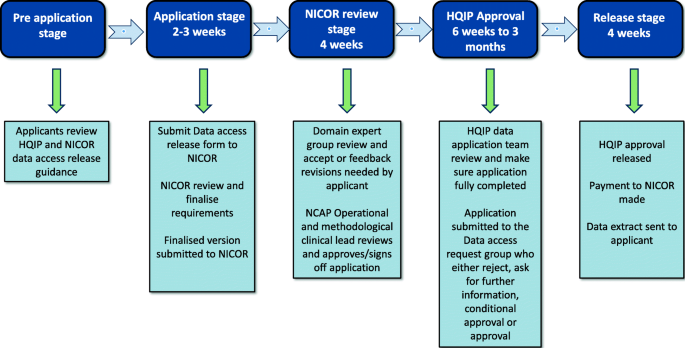
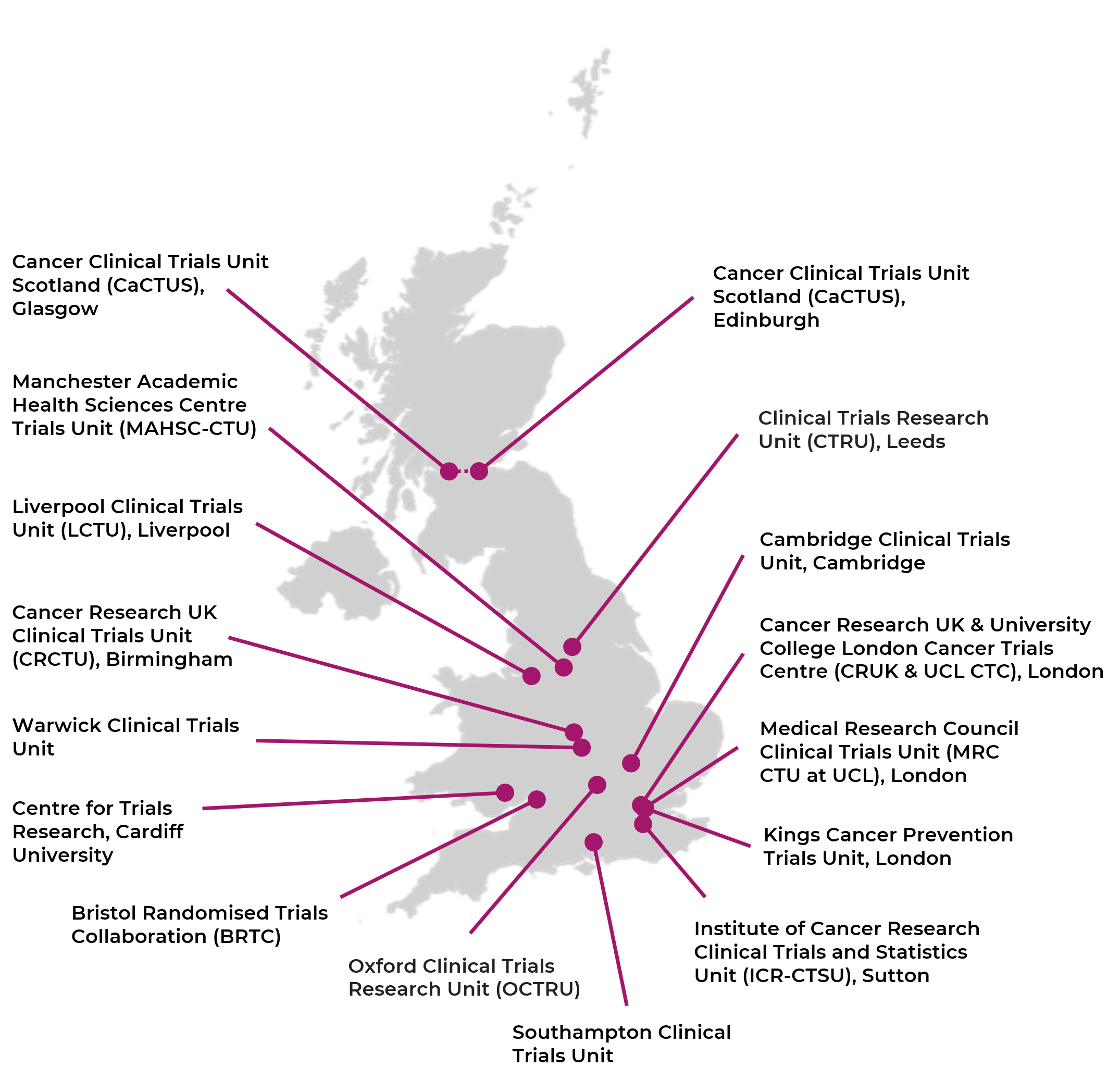
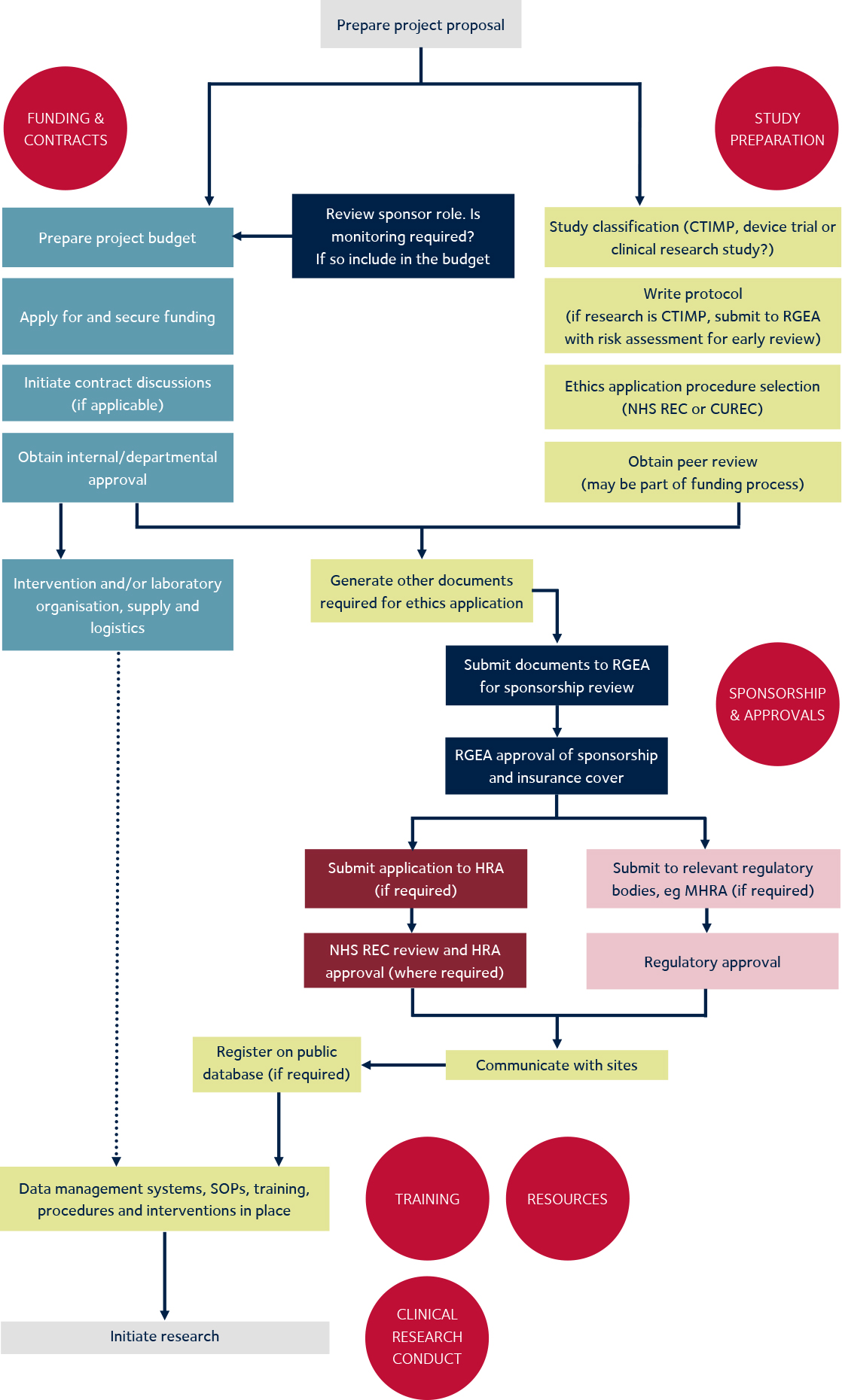
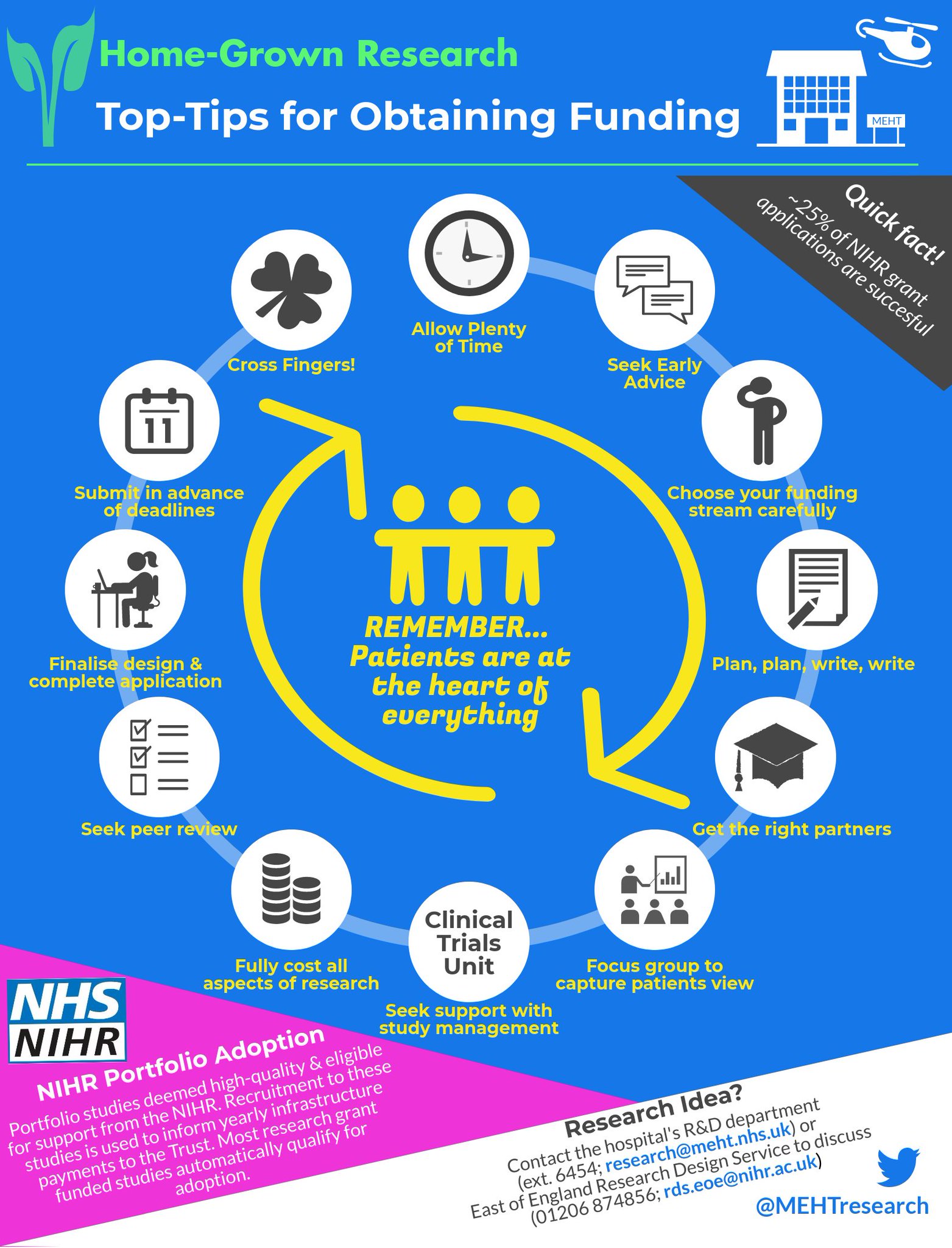
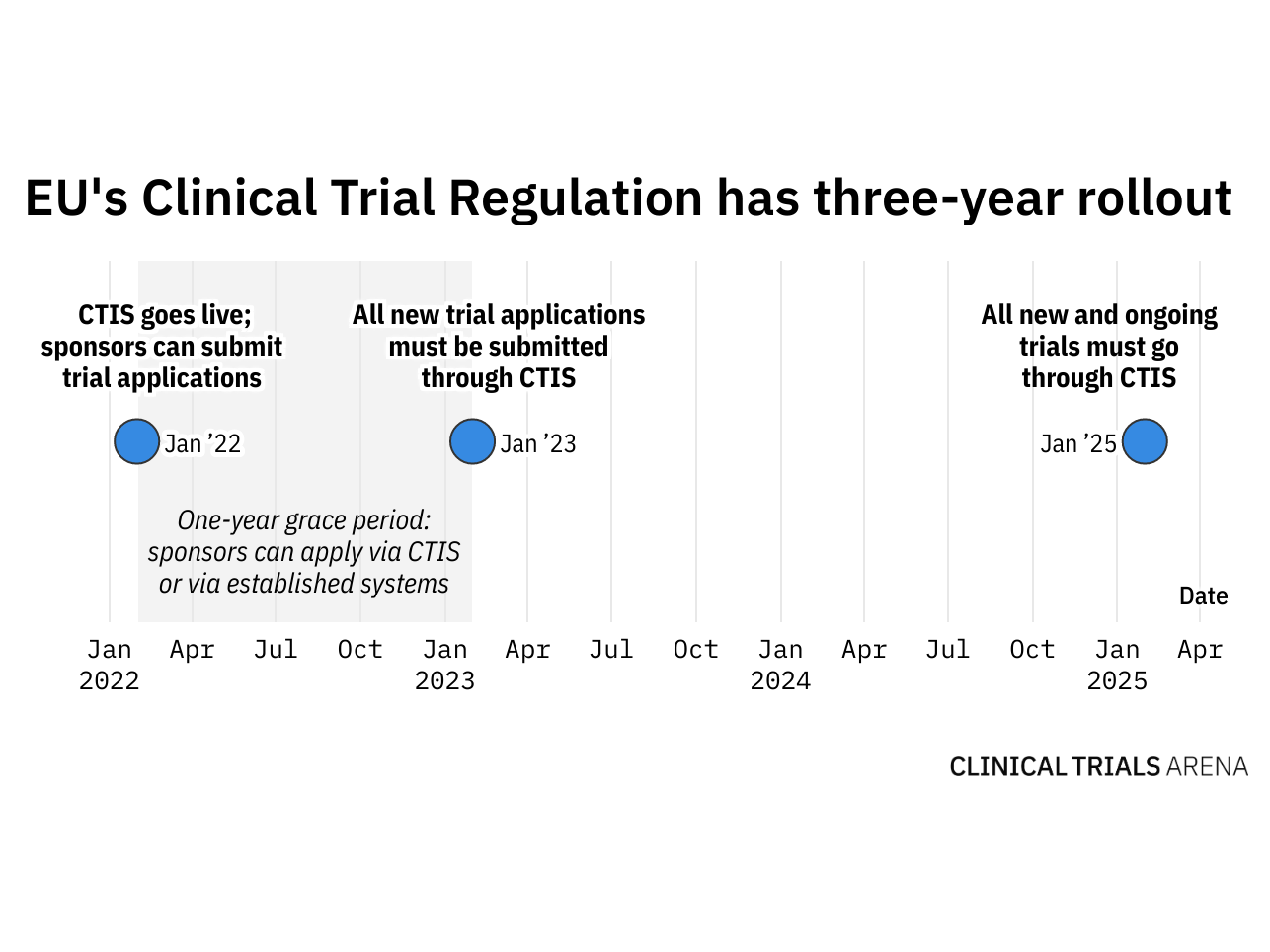
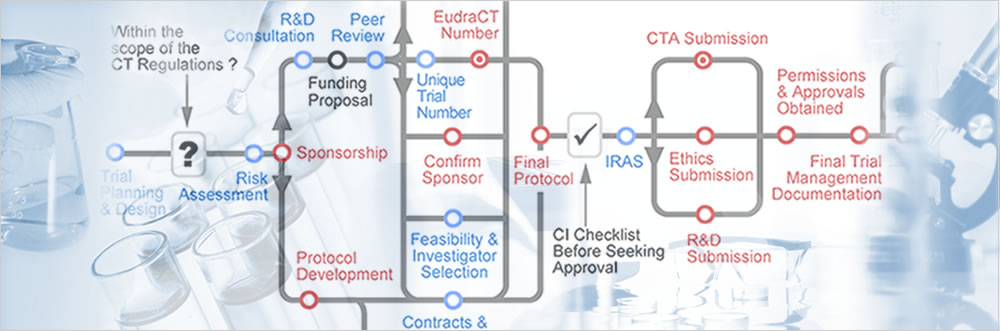
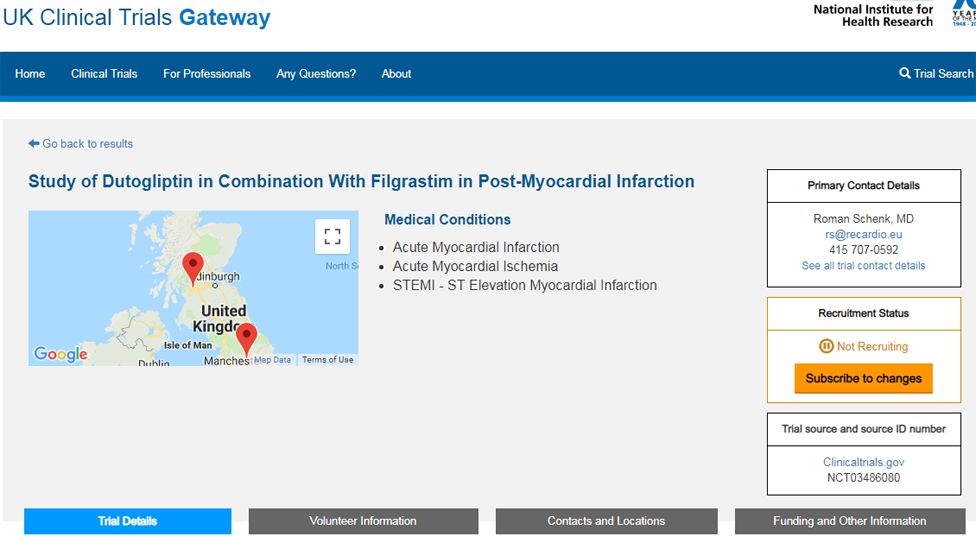
The Use of Routinely Collected Data for Interventional Research in Secondary Care: A feasibility evaluation of a multi-centre ra

CambridgeATS on Twitter: "Next G2T webinar on 8 September, 4pm BST! Dr Tom Oakley, VP of EU Regulatory at ProPharma Group, will talk about the role of regulators in developing new medicines.

Avoiding waste in research: the role of public involvement Iain Chalmers Coordinator, James Lind Initiative 'Putting people first in research' INVOLVE. - ppt download